Novel Olefin Reductive Hydrocarbonation Reaction
A novel study of olefin hydrocarbonation and its applications has been achieved by Prof. FU Yao's research group in Department of Chemistry & Hefei National Laboratory for Physical Sciences at the Microscale. This work presented olefin hydrocarbonation with aryl or alkyl electrophiles and its applications for the modification of complex natural products. The proposed concept 'olefin replacing the traditional organometallic reagents' opened up new ideas for metal-catalyzed cross-coupling reactions as well as provided novel conversions for olefins. Nature Communications published the finding on April 2th (DOI: 10.1038/ncomms11129) with the title of 'Practical carbon-carbon bond formation from olefins through nickel-catalyzed reductive hydrocarbonation'.
Olefins are important synthons in organic chemistry as well as fundamental raw materials for petrochemical industry. Olefin groups are also common in natural products and complex drug molecules, such as Cinchonidine, Vitamin D2 etc. Many metal-catalyzed olefin conversion reactions are also world famous name reactions, such as Heck reaction etc. Recent years, significant progress has been achieved in formation carbon-carbon bonds, which provide general and efficient strategy for the synthesis or modification of complex molecular. However, for a long time, the utilization of moisture and air sensitive organometallic reagents tend to be predominant toward these bond forming reactions. Utilization of inexpensive and readily available olefins to replace these organometallic reagents for the construction of carbon-carbon bonds is not only a novel concept, but also a practical method.
FU's research group reported an example of nickel-catalyzed olefin reductive cross-coupling reaction. In the presence of silane, olefins could be recognized as equivalent to alkyl organometallic reagents. This reaction utilized olefins as direct chemical input, could overcome the limitation cased by the using of traditional organometallic reagents. Furthermore, this reaction has excellent functional group compatibility, thus provides efficient method for the modification of natural products. For example, Vitamin D2 could be successfully converted with high chemo-selectivity in the presence of internal alkene and 1,3-diene groups. Quinine and its derivatives have wide applications as important phase transfer catalysis. This olefin hydrocarbonation reaction could achieve the coupling of quinine and fructose derivative in a convergent fashion which could provide novel and potential phase transfer catalyst. In addition, transformation of simplest ethylene gas was also investigated. The olefin hydrocarbonation process was realized under ordinary pressure using ethylene gas as simple and effective carbon sources.
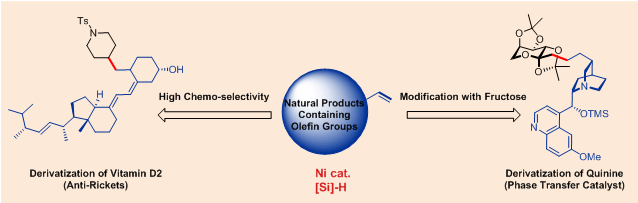
Figure 1. Modification of natural products containing olefin groups. Image by LU Xi.
The work was accomplished with the cooperation of Professor LIU Lei's research group in Tsinghua University. And the project was supported by the National Basic Research Program of China (973 Program) and the National Natural Science Foundation of China (NSFC).
Publication link: http://www.nature.com/ncomms/2016/160401/ncomms11129/full/ncomms111.html
QQ图片20160406140621.png
Back
