Nickel single atomic material for carbon dioxide reduction has made new progress
Recently, new progress about the Ni single atom sites used in CO2 electroreduction was made by the Prof. Yuen Wu’s group (corresponding author) and Prof. Tao Yao’s group from University of Science and Technology of China and Prof. Yadong Li from Tsinghua University. Related paper entitled “Ionic Exchange of Metal–Organic Frameworks to Access Single Nickel Sites for Efficient Electroreduction of CO2” was published on the J. Am. Chem. Soc. (2017, DOI: 10.1021/jacs.7b02736). The first author of the paper is the postgraduate of the University of Science and Technology of China Changming Zhao and undergraduate student Xinyao Dai.
Electroreduction of CO2 into value-added products is an effective approach to remit the environmental and energy issues. Because of the high ratio of low-coordinated metal atoms and technically uniform structure, single-atom catalysts have shown great potential as ideal catalysts for some chemical transformations. However, not only the universality of synthetic methodology for single-atom catalysts but also accurate control over the microstructures still need improvement because of the high mobility and diffusivity of subnanometer species. Nevertheless, the electroreduction of CO2 catalyzed by single-atom materials has seldom been studied.
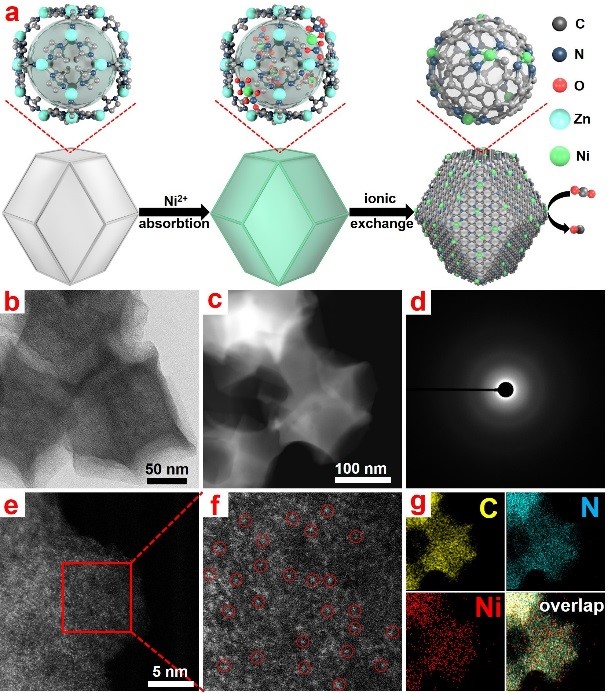
Recently, the authors report a successful synthesis of Ni single atom sites through a double-solvent approach. The Ni precursor could be confined within the pores of ZIF-8 and the Ni single atom sites formed during the pyrolysis. The isolated Ni2+ ions were stabilized by N coordination and further reduced by the surrounding carbon, this unique structure exhibit highly CO2 electroreduction ability and a long-term stability. The current density catalyzed by Ni SAs/N-C reached a high value of 10.48 mA cm−2, highly Faradaic efficiency of 71.9% and maximum Turnover frequency of 5273 h-1 at −1.0 V.

The results of this study not only pioneered the first generation of catalysts for the electroreduction of carbon dioxide, which provides a new idea for the design of reactive sites for the electrocatalytic reduction of carbon dioxide, which plays a crucial role in the study of its reaction mechanism. This work also provides a universal method of synthesizing single atom sites catalysts and expects to bring new enlightenment to the field of single atom synthesis.
This work was supported by the China Ministry of Science and Technology under Contract 2016YFA (0202801) and the National Natural Science Foundation of China (21522107,21671180, 21521091, 21390393, and U1463202) and funding from Collaborative Innovation Center of Chemistry for Energy Materials.
http://pubs.acs.org/doi/abs/10.1021/jacs.7b02736
Back
