Recent advances in N-coordinated dual-metal sites for H2/air fuel
Recently, iChEM researchers, Prof. Yuen Wu’ (USTC) and Prof. Chun-Ran Chang’s (Xi’an Jiaotong University) groups developed a host-guest strategy to construct a novel electrocatalyst with Fe-Co dual sites embedded on N-doped porous carbon and tested its activity for oxygen reduction reaction in acidic electrolyte. The fuel cell test revealed this (Fe,Co)/N-C outperforms most of the previously reported Pt-free catalysts both in H2/O2 and H2/air conditions. This work “Design of N-Coordinated Dual-Metal Sites: A Stable and Active Pt-Free Catalyst for Acidic Oxygen Reduction Reaction” was published in J. Am. Chem. Soc. (J. Am. Chem. Soc., DOI: 10.1021/jacs.7b10385). The first author is Jing Wang, PhD student, from University of Science and Technology of China.
Nowadays, energy consumption and sustainability are one of the most important challenges due to the rising demand and climate change. Fuel cells can convert chemical energy into electric energy with hydrogen as fuel and air as oxidant, generating electricity, water, and heat. Therefore, fuel cells are expected to have great potential for widespread applications on the basis of their high efficiency, no environmental pollution, and unlimited sources of reactants. It is expected that the dual-metal sites electrocatalysts would optimize the activity, stability and selectivity by tuning the well-defined metal active centers. Replacing the Pt-based electrodes with our developed catalyst, the cost of whole fuel cell stack could be reduced from US$52kW−1 to US$43kW−1, and this H2/air single fuel cell reaches a peak power density of more than 505 mW cm-2, ~76 % the power density of commercial Pt/C.
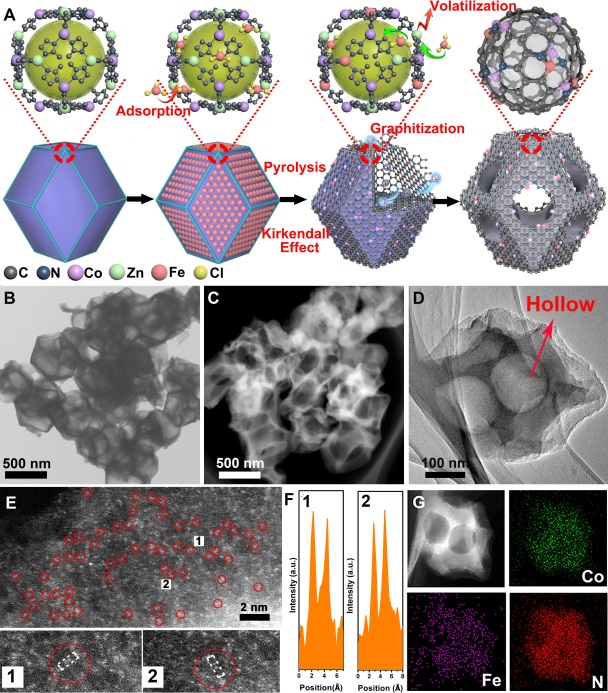
Prof. Wu’s groups report on a host-guest design of hollow carbon-derived material with porphyrin-like Fe-Co dual sites ((Fe,Co)/N-C), which is based on the precise control over the bonding between the Co nodes (host) and adsorbed Fe ions (guest) within the confined space of metal-organic frameworks (MOFs). The Fe3+ moieties were gradually reduced by the as-generated carbon and bonded with the neighboring Co atoms. More importantly, the adsorbed Fe species also served as the catalyst to modulate the geometric structure of carbon support. That is, catalyzed by Fe species, the N-doped graphene was generated by releasing C and N fragments. The Fe species would accelerate the decomposition of Metal-Imidazolate-Metal linkages and enable the generation of voids inside the MOFs. Finally, the continuous decomposition and graphitization would result in the interior cavities and size enlargement, which coincided well with the Kirkendall effect. The conclusion for the structure of Fe-Co dual atoms can be positively predicted from the evidence in synthetic strategies, EXAFS results, HAADF-STEM, and Mössbauer spectroscopic analysis. The density functional theory calculations revealed the dual sites is favored for the activation of O-O, which is crucial for four-electron oxygen reduction process. This well-defined (Fe,Co)/N-C catalyst showed superior ORR performance, with comparable onset potential (Eonset, 1.06 V vs. 1.03 V) and half-wave potential (E1/2, 0.863 V vs. 0.858 V) than those of the commercial Pt/C. Remarkably, the best (Fe,Co)/N-C catalyst displayed a robust long-term durability even after 50,000 cycles in rotating disk electrode test. To date, the maximum initial peak power of H2/air fuel cell delivered by the best non-precious catalyst is only 0.41 W cm-2. In the present work, our (Fe,Co)/N-C catalyst demonstrated a substantial increasement in the initial peak power to a record of 0.51 W cm-2, which is 20% higher than the best non-precious catalyst, reaching ~76% than that of commercial Pt/C. At a constant-current operation test, the working voltage loss was negligible after 100 h.
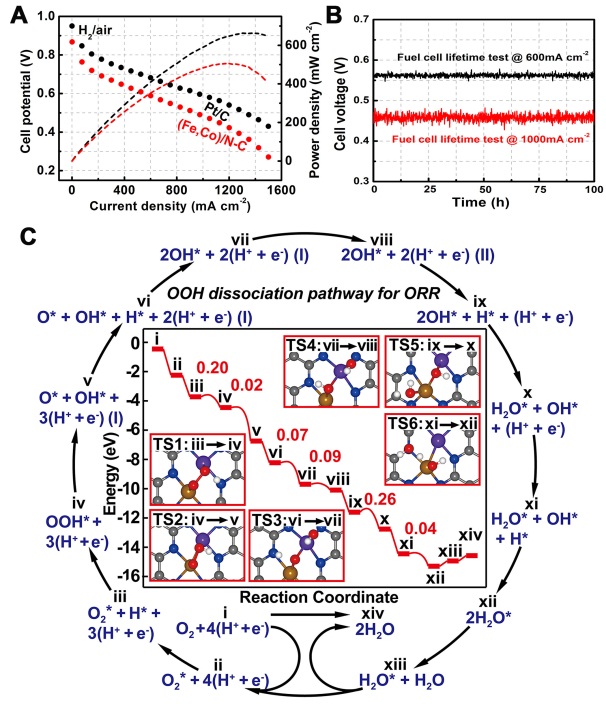
This is a major breakthrough in the field of fuel cells by employing the (Fe,Co)/N-C as a cathode catalyst for fuel cells, which would greatly reduce the cost of whole fuel cell stack. Thus, this work shows promise to greatly enable large-scale commercialization of fuel cell vehicles.
This work was supported by National Key R&D Program of China 2017YFA, and the National Natural Science Foundation of China.
论文链接:http://dx.doi.org/10.1021/jacs.7b10385
Back
