Progress in design of cathode electrocatalyst for proton exchange membrane fuel cell
Recently, the research group of Prof. Jie Zeng from School of Chemistry and Materials Science & Hefei National Laboratory for Physical Sciences at the Microscale has made significant progress in the design of efficient nanocatalysts toward oxygen reduction reaction (ORR), a cathodic reaction of fuel cells, with the collaboration of Prof. Hongwen Huang from Hunan University. Researchers designed a new type of catalyst by combining the merits of high utilization efficiency of Pt atoms, anisotropic one-dimensional nanostructure, and PtNiRh Trimetallic. This work was published on Journal of the American Chemical Society (J. Am. Chem. Soc.2018, 140, 16159-16167), titling" One-Nanometer-Thick PtNiRh Trimetallic Nanowires with Enhanced Oxygen Reduction Electrocatalysis in Acid Media: Integrating Multiple Advantages into One Catalyst".Graduate student Kan Li and Dr. Xingxing Li contributed equally to this work.
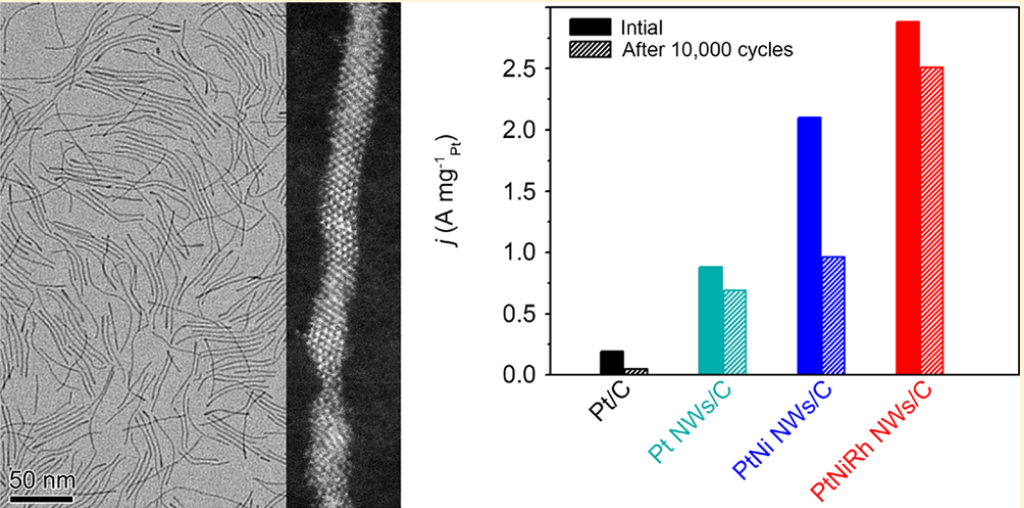
Structure of PtNiRh Trimetallic Nanowires and their catalytic performance in oxygen reduction reaction
Proton exchange membrane fuel cells (PEMFCs) have beenrecognized as a promising clean energy conversion technologyfor efficient power delivery in transportation and mobile devices. However, the commercialization of such technology has so far been hampered by the prohibitive cost of associateddevices, because a large amount of precious platinum (Pt) is required as catalyst to mitigate the sluggish kinetics of theoxygen reduction reaction (ORR) at the cathode. Onesolution to lower the cost is to reduce the usage of Pt catalyst in PEMFCs by improving the mass activity toward ORR, besides, another method to improve the massactivity is to boost the specific activity of a catalyst. Althougha number of Pt-based catalysts with excellent mass activitieshave been successfully reported, most of these catalysts sufferedfrom the relatively mediocre durability. Theinsufficient durability of these Pt-based catalysts is ascribed tothe thermodynamically unstable structures that are generallyneeded for high mass activities, so it seems that there is aconflict between high mass activity and superior durability.
Faced with the challenges, researchers designed and synthesized a remarkable Pt-based catalyst by combing the features of high utilization efficiency of Pt atoms, anisotropic one-dimensional nanostructure, and PtNiRh Trimetallic Nanowires. The mass activity and specific activities of the produced PtNiRh Trimetallic Nanowiresare 15.2 and 9.7 times higher than that of commercial Pt/C catalyst, respectively. Moreover, even after 10,000 cycles of accelerated durability test in O2 condition, the Rh-doped Pt nanowires/C catalyst exhibits a drop of 12.8 % in mass activity, against a big decrease of 73.7% for commercial Pt/C.
This work was supported by CAS, MOST of China, National Natural Science Foundation of China, and China Postdoctoral Science Foundation.
Source: https://pubs.acs.org/doi/abs/10.1021/jacs.8b08836
Back
